|
WHAT IS LIPO SPRAY? Tablets, liquid, two-piece gelatin capsules and soft-gelatin capsules are all designed to be absorbed through the digestive tract. Revolutionary LipoSpray technology offers a unique alternative to conventional administration of nutritional supplements-simply, conveniently and effectively eliminating the need for pills, tablets, and liquids, while providing improved results for consumer. With LipoSpray, the liposomal suspension is sprayed into the mouth and under the tongue. The liposomes penetrate the mucosal tissue of the mouth, and the nutrients are released from the liposome into the bloodstream, thus distributing the nutrients throughout the body in minutes. This path bypasses the digestive system. Conventional pills, tablets and liquids must travel through the digestive system in order to deliver active ingredients to the body.
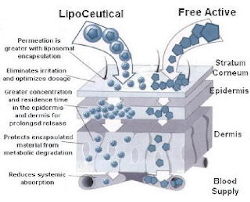
 WHAT IS LIPOCEUTICAL?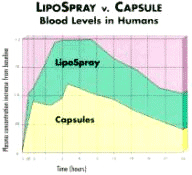 LipoCeuticals are liposomes in a multiphase system that contain an active ingredient in each phase. The ability to encapsulate a variety of lipophilic and hydrophilic ingredients peptides and proteins will give you the advantage needed to enhance delivery and improve product performance.
Back to LIPOSOME SPRAYS HGH and ENHANCE
|
